 |
| |  | |
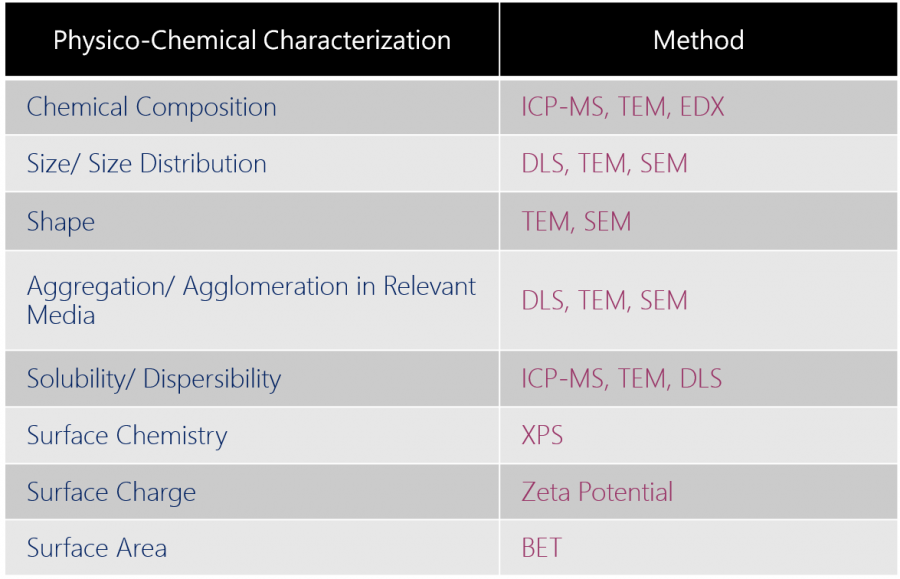
| Following the recommendations in ISO/TR 13014, Bio MA-Tek established comprehensive in house solutions that answer the needs addressed in the report. | ||
 |
| Reference 1. ISO/TR13014:2012 “Nanotechnologies-Guidance on physico-chemical characterization of engineered nanoscale materials for toxicologic assessment”. 2. Overview of CDER Experience with Nanotechnology-relative Drugs, US FDA August 2012. 3. Requirements on Measurements for the Implementation of the European Commission Definition of the Term “Nanomaterials”, EU/JRC July 2012. |